Device Reimbursement Commercial Services Research Services Selling Into the Australian Market International Trade Organisations
Device Reimbursement
If you can get your product reimbursed, it will be a lot easier to sell.
The Australian Market is estimated to be worth $US 5-6 billion.
Of this, approximately $US 2 billion is reimbursed through health insurance.

Fixed Price Reimbursement Analysis
Find out if your product is eligible for reimbursement with our low cost fixed price package. You’ll know exactly how much your product will receive, making it easy to work out your potential distribution costs and margins before you file for all the regulatory approvals.
Fixed Price Reimbursement Listing
If your device is eligible, and you’re satisfied with the amount you will receive and want to bring your product to market, we offer a low price, fixed cost listing service. In most cases we can have your device reimbursed by all the Australian Health Insurers within 12 months. Insurers within 12 months.
Regulatory Approval
We have different packages to suit your needs.
Before your device can be sold in Australia, it needs to be approved by the Therapeutic goods Administration (TGA), and listed on the Australian Register of Therapeutic Goods (ARTG).
You will need an Australian sponsor, along with 24 hour contact phone numbers and you need to meet various obligations with regards to recalls, packaging and storage.

Regulatory Approval
If your device is ready to be taken through the regulatory hurdles, we can complete and make all the necessary submissions and follow through with the TGA, making sure any potential issues are dealt with before they become unnecessary problems.
We can offer you Australian Sponsorship if required.
Fixed Price Regulatory Analysis
Prior to commencing any filings, and running up large fees, let us conduct a fixed price analysis to let you know if there may be any problems gaining regulatory approval for your technology, what class of devices it will fall into, and whether the information and technical files you already have will be enough to get your device approved.
We’ll work out the quickest and cheapest way to get your product approved.
Commercial Services
There is more to the success of a device than simply gaining “regulatory approvals”.
Our commercial services will help you see through the often confusing and conflicting accounts of how to go about selling devices into the Australian market.
Using our expert local knowledge and network of industry professionals, we can provide you with the information you need to understand the local market, how big is it in relation to your product, who are the buyers, who are the decision makers, what the competition is doing, and help you determine the best way to sell your devices into the Dynamic Australian Market.
Let’s say you want to bring a new type of spinal fusion device to the Australian market…
Know the details
WHAT, WHO AND WHERE IS YOUR REAL MARKET?
…and how big is it?
IS IT EVERY PATIENT UNDERGOING A SPINAL FUSION?
Is it every hospital undertaking spinal procedures?
OR IS YOUR MARKET A HANDFUL OF SURGEONS WHO PERFORM A SPECIFIC TYPE OF SPINAL FUSION PROCEDURE?
How many of those specific procedures are performed each year?
WHAT MAY INITIALLY SEEM LIKE A LARGE MARKET…
…may in fact be a very small one, controlled by half a dozen individuals.

Know your market
WHAT IS YOUR MARKET?
How big is your market?
WHO ARE THE DECISION MAKERS?
…and how many are there?
WHICH HOSPITALS WILL USE YOUR PRODUCTS?
ARE THEY PERFORMED IN ONLY ONE HOSPITAL IN ONE STATE?
ARE THEY PERFORMED IN 4 HOSPITALS IN 4 STATES?
Requiring four times the investment in representative support and instrument sets?
We’ll keep drilling down, so you’ll know ahead of time what, who and where your market is, and be able to develop corresponding strategies and budgets to allow you to quickly target the sub-markets with the highest potential.
Competitor Analysis
Your competitors have 100% of the market – how do you plan to win market share from them?
You need to know who your competitors are, and how your competitors promote their products. How they sell a competing product in Sydney may be different to how they sell it in Perth. Do they have customers locked into contracts, do they supply capital equipment free as part of a usage deal? Do they lock hospitals into bundle deals or give rebates, effectively reducing the price you thought you would receive?
Go into the market with your eyes open, knowing ahead of time what you need to do to compete.

Research Services
Device Services Australia specialises in the commercial understanding of the medical device market.
We believe too much weight is put onto “Regulatory Approvals” and not enough focus goes into determining ahead of time how a new product or technology will actually be sold, and who the buyer really is.
Of course it is true that you won’t be able to sell a device without the necessary approvals, but that alone won’t move a product. After all, how many investor presentations have you been to over the years where a new technology is being developed, and watch the momentum (and share price) build as the device passes the different regulatory processes until it gains the final ‘Regulatory Approval’ only for the device to be a sizzling commercial failure?
Our research reports are designed to be easily read and understood by the non-medical professional. We assume your audience knows nothing about the market you’re interested in, and we take a step by step approach to explaining the entire device market, how it works, who chooses the devices, who pays for them, and narrowing down the focus to your chosen device or technology.

Informative Research Report
You can expect to know by the end of our Research Report exactly how big your market is, who will be using it, which hospitals will stock it, and how much you can sell it for. You will know whether your device or technology will be eligible for third party payment, and if not, the steps you might take to ensure eligibility.
We’ll examine the level of difficulty and resistance a new entrant might have in a particular market, how much support and training will users need, ie a couple of training sessions by a company rep, or ongoing attendance every time the device is used?
This has implications for the size of the sales force required and the ability to sell it in regional areas.
We’ll work out the quickest and cheapest way to get your product approved.
Selling Into the Australian Market
How do you find and qualify the best distributors or agents for your products?
You’ve got the regulatory approvals, national reimbursement and you’ve done all your research on the market and you feel fully prepared, but …. it will all fall over if the organisation selling your device just isn’t effective.
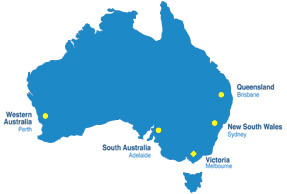
Distributor Search and Qualification
The Australian market has some unique challenges when compared to other international markets. Although the market size is large based on the spend compared to population, the market is concentrated in 5 geographic areas, Sydney, Melbourne, Brisbane, Adelaide and Perth, all of which are between 1000km -4000km apart. Few distributors operate directly in all those markets.
We can determine where in Australia your product needs to be represented. If it’s a specialised device used in paediatric neurosurgery, there may only be 4 hospitals in Australia able to perform the surgery that uses your product. Instead of a national distributor which sells everything, you may get better representation through specialist independent sales agents who have the personal relationships with end users to be seen and taken seriously.
Or perhaps your product is an electrosurgical device that can be used in every operating theatre in Australia, in which case you may want as many non-specialised reps as you can get covering your product across Australia.
When it comes to qualifying distributors, we examine how a distributor incentivise their sales staff, the product mix of their representatives, and the overall effectiveness of the distributors organisation and processes.
International Trade Organisation
Your organisation may be tasked with looking for new markets for your country’s industry to enter, and you are interested in learning whether the Australian market may be suitable for you.
We can provide you with a comprehensive analysis of the Australian market specific to your technologies or products, and act as a liaison between yourself and the Australian Government and industry.
Whatever your needs, we can offer professional and experienced guidance, and help you take your industry into a stable, high potential market.
Contact Us
Do not hesitate to get in touch. One of our friendly staff will answer any queries you may have. We look forward to working with you.
Suite 2201, Level 22, Tower Two,
Westfield Bondi Junction
101 Grafton St
Bondi Junction NSW 2022
+61 414 389 418
info@deviceservicesaustralia.com.au